| In brief, if
your products need not to be conducted clinical trials, and the
Application Dossier are appropriate, you shall obtain IDL within
about 9-12 months after submission. If clinical trials must be
conducted, the general procedure is:
Step 1.
Submitting an application and if the
Application Dossier are appropriate, you shall obtain Clinical
Trials Permission (CTP) within about 10-12 months after submission

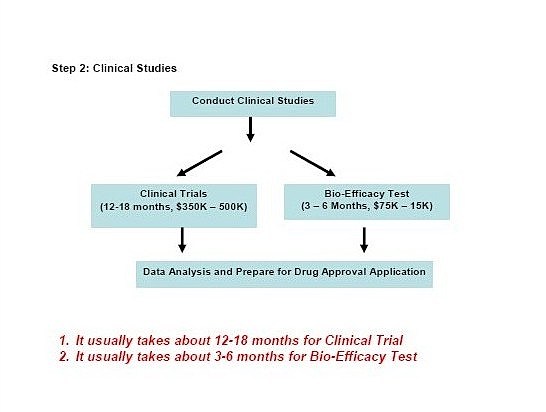
Step
2.
Conducting the clinical trials, if just
carry out bioequivalence trials, it might be take 3 to 6 months, but
other clinical trials might be take more than 1-1.5 year to
complete.
Step 3.
Submitting the Application Dossier (include the reports of
clinical trials) and if the Application Dossier are appropriate, you
shall obtain IDL within about 12-18 months after submission Please
contact us for details about this and for more information
including, we shall send the further information and our quotation
to you after receive the introduction of your products.
 |